Necrotizing Enterocolitis
Chief Editor- Suraj Gupte, Authors-Dinesh Kumar Chirla
Part 1 - Spotlight: Neonatal Nutrition
Recent Advances in Pediatrics—25: Perspectives in Neonatology
INTRODUCTION
Necrotizing enterocolitis (NEC) is the most common gastrointestinal emergency in neonates. It is a disease of unknown etiology with multi- factorial pathogenesis. In 1823, Charles Billard described what probably could be the first case report of necrotizing enterocolitis (NEC) as gangrenous enterocolitis in a small weak infant with infection, inflammation, and necrosis of the gastrointestinal tract (GIT).1 In 1965, Mizrahi and colleagues2 first used the term necrotizing enterocolitis to describe a clinical syndrome consisting of vomiting, abdominal distention, shock, intestinal hemorrhage and perforation. In 1978, Bell and colleagues3 classified NEC into 3 stages based on the severity of the clinical presentation and recommended treatment

Fig. 22.1 (A) Only 15% of term (T) and late preterm (LPT) infants developed NEC in this cohort of 202 infants with NEC. EP, extremely premature; MP, moderately premature; VP, very premature. (B) Age of onset of NEC is inversely related to PMA at birth. (Data from Sharma R, Hudak ML, TepasJJ 3rd, et al. Impact of gestational age on the clinical presentation and surgical outcome of necrotizing enterocolitis. J Perinatol 2006;26:342–347)
strategies. Despite incremental advances in our understanding of the clinical presentation and pathophysiology of NEC, universal prevention of this serious and often fatal disease continues to elude us even in the twenty-first century.
Figure 22.1 shows the impact of gestational age on the clinical presentation of necrotizing enterocolitis.
INCIDENCE
The incidence of NEC is reported between 0.3–3/1000 live births. The risk of developing NEC is inversely related to gestational age at birth with the extremely premature infant at greatest risk.5 More than 85% of all NEC cases occur in very low birth weight (VLBW) infants (<1500 g) or in very premature babies. Multicenter and large population based studies demonstrate that it is prevalent in 7 to 11% of VLBW infants. It is less common in term and late preterm infants. Only 7 to 15% of all NEC cases occur in term or late preterm infants. In term infants, NEC is commonly associated with congenital heart diseases, such as hypoplastic left heart syndrome and coarctation of the aorta, which result in intestinal hypoxia and/or hypoperfusion.
RISK FACTORS
The pathophysiology of NEC in very premature (VP) infants is not completely elucidated. Compared with NEC in term and late preterm infants in whom hypoxia-ischemia is a common precursor, recent advances in our understanding of NEC at the molecular level suggest that an inflammatory response in VP infants plays the inciting or dominant role in the pathogenesis of NEC.
Previously held beliefs that low Apgar scores, umbilical catheterizations, episodes of apnea and bradycardia, respiratory distress syndrome, anemia, hypothermia, hypoxic-ischemic events, hypotension, and the use of vasoactive agents such as Indocin and pressors are important contributing causes of NEC in premature infants have not been supported by large epidemiologic and more recent clinical studies.4
Although hemorrhagic-ischemic necrosis is the terminal manifestation of NEC in premature infants, the interaction among milk substrate, microbes, and the immature host immunologic system is now thought to be the key in initiating the pathogenesis of NEC.
Microbial Mucosal Interaction and NEC
Microbial components, such as lipopolysaccharides (LPS), lipoteichoic acid (LTA), formylated peptides, and flagellin, serve as microbial-associated molecular patterns (MAMPs) and signal pattern recognition receptors (PRRs), include toll-like receptors (TLRs), formylated peptide receptors (FPRs), or nucleotide-binding oligomerization domain-like receptors (NODs). Integration of these signals evokes cellular outputs based on the initial perception of the triggering organism. Activation of these PRRs initiates regulatory pathways, including mitogen-activated protein kinase, nuclear factor kB (NFkB), and caspase-dependent pathways.
These interrelated complex pathways determine mucosal and submucosal responses. Based on the perception of interrelated signals, the response can be cytoprotective (i.e. promoting growth and repair) or it can be destructive if apoptosis or inflammatory responses is triggered. The terminal event in NEC is hemorrhagic ischemic necrosis, which is the consequence of a dysregulated inflammatory response mediated by endogenous factors that include platelet-activating factor (PAF), proinflammatory cytokines (such as tumor necrosis factor, interleukins 1 & 6 [IL-1, IL-6]), chemokines (MIP-2/ CXCL2), NF-kB, and the complement system.6
Because of the relative immaturity of key gastrointestinal functions, such as digestion, absorption, motility, and abnormalities in immune responses, a premature newborn is exquisitely vulnerable to gastrointestinal injury. Premature infants are exposed shortly after birth to a massive microbial antigenic challenge that may be distorted by frequent and prolonged use of antibiotics. As a result, the GIT of premature infants gravitates toward mounting an inflammatory response rather than establishing tolerance (T-helper-1 response).
Hypoxic Ischemic Mechanism
Under normal conditions, a state of high intestinal blood flow and low resting vascular resistance is maintained by nitric oxide.7 Impaired endothelial function or elaboration of proinflammatory mediators may alter the balance between vasoconstriction (as mediated by endothelin-1) and vasodilatation (as mediated by nitric oxide) and lead to a relatively ischemic state.
Infectious Agent
Despite 4 decades of exhaustive search, no consistent single microbial species has been isolated from infants with NEC.8 Enterobacteriaceae sp and Klebsiella are the most common, followed by Staphylococcus sp and Clostridium sp. Outbreaks of NEC linked to consumption of formula contaminated by Enterobacter sakazakii and breast milk contaminated by Staphylococci have occurred. Although bacteria are most commonly associated with NEC, several enteric viruses (rotavirus, echovirus, coronavirus, torovirus, norovirus [NoV]) and Candida sp have also been described.
Generally, NEC occurs sporadically but may also occur in clusters or outbreaks. Temporal clustering of such outbreaks and their cessation with the implementation of infection control measures supports an association of these outbreaks with a single transmissible agent during a given outbreak.9
Transfusion and NEC
In recent years, several reports have attempted to establish a causal relationship between blood transfusion (BT) and NEC. These studies propose that BT for anemia of prematurity in relatively stable growing premature infants increases the risk of late-onset NEC.10
Antibiotics
Intestinal colonization with commensal Bifidobacterium and Lactobacillus species reduces the risk of NEC. The frequent and prolonged use of antibiotics results in an overgrowth of potentially pathogenic species. A recent clinical study found that exposure to broad-spectrum antibiotics for more than 10 days increased the risk of NEC by nearly threefold.
Feeding
Feeding practices have long been implicated in the development of NEC. This theory has been difficult to clearly elucidate, because feeding practices have varied significantly with time, as well as from NICU to NICU.
Rate of feeding: Several case-control and retrospective studies have reported that feeding or increasing feeds too rapidly seems to increase the risk of NEC. However, Cochrane systematic reviews examining feeding practices including delayed introduction of enteral feeds and slow advancement of enteral feed volumes have failed to show a significant association.11 In clinically stable very low-birth-weight (VLBW) infants, early introduction of progressive feeds and advancement of feeds at a faster rate (30–35 mL/kg/d) is safe and does not increase the incidence of NEC.
Standardized feeding protocols or feeding guidelines, which typically involve initiation of feeding based on gestational age of the infant and slow gradual advancement of both volumes and concentration, have been shown to have a lower incidence of NEC compared with nonstandardized initiation
of feeds.12 In addition, the use of feeding guidelines has been shown to improve growth, reduce the length of stay, and lower hospital costs.
Type of milk: Meta-analyses have suggested that the use of human milk reduces the incidence of NEC, and that fortification of milk does not increase the incidence of NEC. A recent prospective, randomized trial comparing the exclusive use of human breast milk with bovine milk-based products found that the incidence of NEC was 77% lower in infants exclusively fed human breast milk and that the incidence of NEC requiring surgical intervention was also significantly lower.13
A recent quality improvement project in California to increase breast milk use in very low birth weight infants resulted in a reduction of NEC from 7 to 2.4%.
The evidence is convincing that human milk feeding, compared with formula feeding, reduces the incidence of necrotizing enterocolitis (NEC) in preterm infants.
Human milk-based fortifier compared with bovine-based fortifier may reduce the incidence of NEC but additional studies are required.
Minimal enteral nutrition (MEN) is a safe alternative to complete fasting before initiation of progressive feedings and does not increase the incidence of NEC in extremely preterm infants. In a feed-intolerant preterm infant without any other clinical and radiologic evidence of NEC, MEN rather than complete suspension of enteral feeding may be an alternative. MEN, otherwise called trophic feeds or gut priming, is usually started within 1 to 3 days after birth with 15 mL/kg/d to 20 mL/kg/d of enteral milk given every 2 to 3 hours. Similarly, a Cochrane review comparing trophic feeding with no feeding or advancing feeding practices showed no consistent results in terms of incidence of NEC, and hence no clear benefit to either practice regarding NEC.14
Continuous Versus Intermittent Feeds
There is no evidence supporting continuous versus intermittent tube feedings in preterm infants.
Aggressive Feeding
In premature infants, aggressive feeding may cause stasis of milk substrate in the lumen of the GIT because of dysmotility.15 Stasis can lead to intestinal dilatation with fluid and gas and possibly to impairment of the intestinal epithelial barrier (IEB). The development of endotoxemia in stable premature infants after feeding and evidence from other studies support that the IEB of premature infants is leakier compared with that of more mature infants. Intestinal dilatation in the presence of abnormal microbial colonization (dysbiosis) can distort normal signal transduction (crosstalk) across the IEB and alter the normal message of growth and repair of enterocytes to one that instead produces excessive inflammation, apoptosis, and necrosis.
CLINICAL PRESENTATION
More recent studies have found that a peak distribution of NEC occurs at 29 to 31 weeks of PMA.5 This shift to an earlier PMA may be a reflection of the current practice to introduce enteral feedings earlier compared with the delayed feeding practices of the 1980s.
NEC varies greatly, infants may have a sudden onset with rapid clinical deterioration or it may evolve slowly over a few days. Consider NEC if any of the following signs are present:
- Non-specific GIT: feed intolerance, abdominal distention, occult blood in stool
- Specific GIT: increased abdominal distention (Fig. 2) with tenderness, abdominal wall edema, decreased or absent bowel sounds, bile stained gastric aspirates, bloody stool. VP infants do not manifest tenderness and guarding unless NEC is advanced
- Systemic: temperature instability, apnea, persistent acidosis, thrombocytopenia, anemia, neutropenia and cardiovascular compromise such as hypotension, oliguria and
INVESTIGATIONS
Laboratory
FBC: Anemia, neutropenia and thrombocytopenia are often seen. The early return of these indices to normal carries a good prognosis.
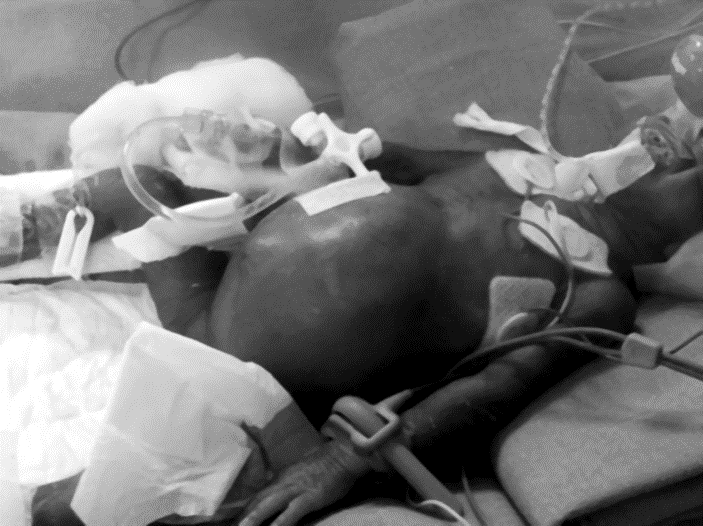
Fig. 22.2 Abdominal distention with shiny skin
- Blood film: Look for evidence of hemolysis and toxic changes
- Electrolytes: In some instances, NEC may present as unexplained hyponatremia.
- Arterial or venous blood gas: For evidence of acidosis, hypoxia or
hypercarbia.
- Coagulation profile: if there is active
- Blood cultures: Concurrent bacteremia and sepsis occurs in 40 to 60% of NEC Bacterial and viral cultures may be helpful but not conclusive.
Imaging
Both a supine AP abdominal and left lateral decubitus X-ray are essential for the diagnosis of suspected NEC. It is important to note that radiological findings associated with NEC are not seen in all infants. They may appear even before clinical signs.
- ray findings include:
- Dilated and thickened bowel loops +/– air-fluid levels
Pneumatosis intestinalis (intramural gas): The radiological hallmark of NEC. Pneumatosis is caused by gas within the bowel wall and may appear linear (like railroad tracks) or circular if gas is subserosal or bubbly if gas is submucosal (Fig. 22.3).

Fig. 22.2 Abdominal distention with shiny skin
- Blood film: Look for evidence of hemolysis and toxic changes
- Electrolytes: In some instances, NEC may present as unexplained hyponatremia.
- Arterial or venous blood gas: For evidence of acidosis, hypoxia or
hypercarbia.
- Coagulation profile: if there is active
- Blood cultures: Concurrent bacteremia and sepsis occurs in 40 to 60% of NEC Bacterial and viral cultures may be helpful but not conclusive.
Imaging
Both a supine AP abdominal and left lateral decubitus X-ray are essential for the diagnosis of suspected NEC. It is important to note that radiological findings associated with NEC are not seen in all infants. They may appear even before clinical signs.
- ray findings include:
- Dilated and thickened bowel loops +/– air-fluid levels
Pneumatosis intestinalis (intramural gas): The radiological hallmark of NEC. Pneumatosis is caused by gas within the bowel wall and may appear linear (like railroad tracks) or circular if gas is subserosal or bubbly if gas is submucosal (Fig. 22.3).

Fig. 22.4 Large pneumoperitoneum, creating football sign in a neonate with NEC
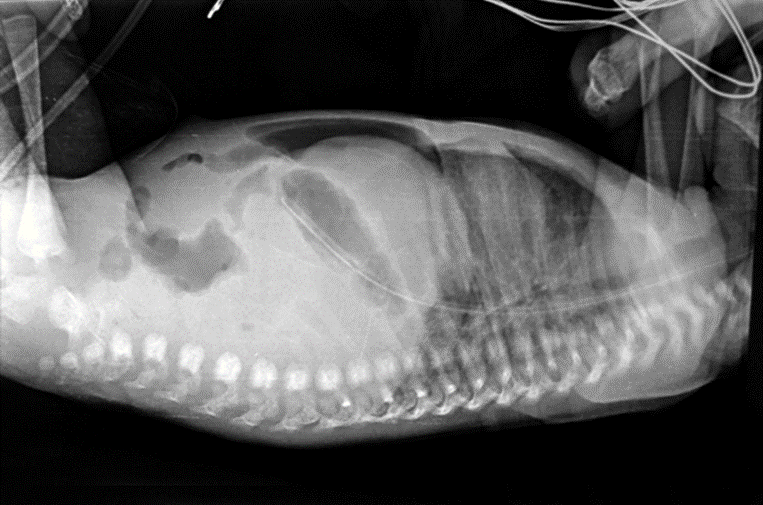
Fig. 22.5 Lateral view showing cross table of pneumoperitoneum in NEC
difficult to visualize on a single plain film. A left lateral decubitus film will allow free air to rise to the top over a nondependent surface, facilitating visualization of an abnormal lucency. Sometimes free air may present as a double-wall sign when the bowel loop is outlined and gas is present along serosal and mucosal surfaces
- Persistently distended loop of bowel
- A persistent, fixed, dilated loop may indicate a necrotic bowel loop
- Portal venous gas: Pneumatosis intestinalis can extend to the portal venous circulation and typically appear as curvilinear lucencies over the hepatic silhouette in a plain radiograph (Fig. 6).
- Gasless
Ultrasound: The role of ultrasonography in the evaluation of NEC is not yet clear. This modality can be used to assess bowel wall viability (using color Doppler), as well as assess bowel wall thickness, peristalsis, fluid collections with presence of particulate debris, pneumatosis, or portal venous gas, and may be useful in cases in which radiographs do not match the clinical picture. Faingold and colleagues used color Doppler sonography and demonstrated 100% sensitivity for free air and absent blood flow (necrotic gut) compared with 40% sensitivity by radiography.16
Contrast studies: These are best avoided during the acute illness as there is a high risk of perforating. Due to the lack of evidence supporting contrast studies during the recovery phase, we suggest they be reserved for when there are concerns of late strictures.
Table 22.1 lists the modified bell criteria for staging NEC.
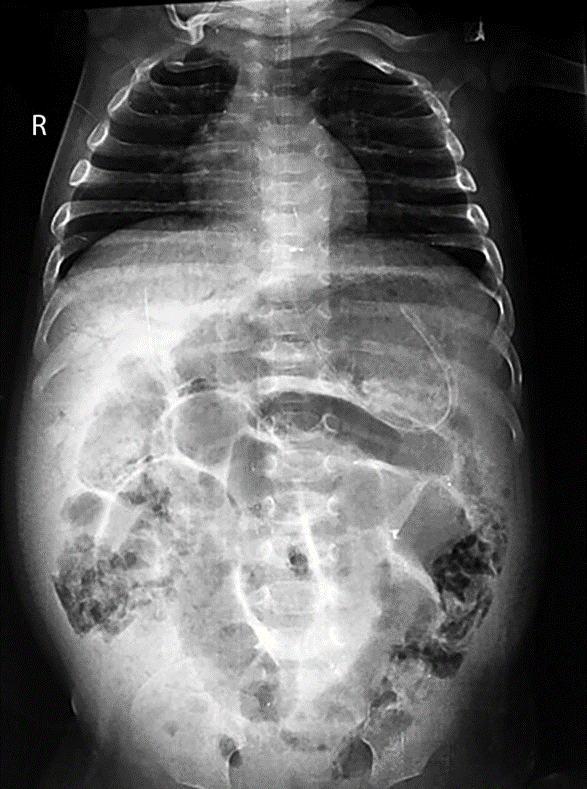
Fig. 22.6 Portal vein gas and pneumatosis intestinalis in NEC
Table 22.1 Modified Bell criteria for staging NEC
Stage | Systemic Criteria | Abdominal Criteria | Radiographic Criteria |
1a: Suspected NEC | Temperature instability, apnea, bradycardia | Increased pregavage residuals, mild abdominal distension, occult blood in stool | Normal or intestinal dilatation, mild ileus |
1b: Suspected NEC | Same as above | Grossly bloody stool | Same as above |
2a: Definite NEC; mildly ill | Same as above | Same as stage 1 plus lack of bowel sounds, possible abdominal tenderness | Ileus, penumatosis intestinalis |
2b: Definite NEC; moderately ill | Same as stage 1 plus mild metabolic acidosis, mild thrombocytopenia | Same as above plus peritonitis, definite abdominal tenderness, possible cellulitis, right lower quadrant mass | Same as above plus possible portal venous gas |
3a: Advanced NEC; severely ill, intact bowel | Same as stage 2b plus hypotension, severe apnea, combined respiratory and metabolic acidosis, disseminated intravascular coagulation, and neutropenia | Same as above with marked tenderness and abdominal distension | Same as above plus ascites |
3b: Advanced NEC; severely ill, perforated bowel | Same as stage 3a | Same as stage 3a | Pneumoperitoneum |
PATHOLOGY
NEC affects all portions of the GIT most commonly the jejunum, terminal ileum and proximal colon. The gut with NEC shows mucosal and transmural coagulation necrosis, hemorrhage, inflammation, ulceration and reparative changes.
TREATMENT PROTOCOL
Most infants with NEC can be managed conservatively but 30–50% will require surgical intervention. As there have been no controlled trials of treatment for NEC that have shown benefit, management is essentially supportive and based on empiric interventions.
Serial physical examinations and investigations should be done to guide treatment. Early surgical consultation is essential.
Bowel Rest and Nutrition
Initial clinical management is directed at prevention of further injury to the gut.
- Cessation of enteral feeding. Conventionally the duration of no enteral feed is usually 10 days. This is empirical with no available evidence to support this. Consider earlier recommencement of feed if gut function is returned to normal, i.e. soft, nondistended, nontender abdomen with normal bowel sounds and minimal gastric
- Gastric decompression with a large bore 8–10F orogastric
- Commencement of intravenous fluids
- Correction of electrolyte disturbances
- TPN should be commenced unless there is severe
- Gradual reintroduction of feed, ideally this should be with breast Following the re-introduction of feeds, up to 10% of infants show increased gastric residuals and abdominal distention.
Intravenous Antibiotics
- Broad spectrum intravenous antibiotic cover against Gram positive and negative organisms should be commenced as soon as the diagnosis is considered. Because of the prominence of anaerobic bacteria the routine inclusion of antianerobic drug is Clinical trials for definitive management regimens are lacking, but therapy should be determined by the sensitivity of local organisms.
- The recommended antibiotic regime based on the prevalence of organism and sensitivity of the unit. Most of the unit will consider Piperacillin/tazobactum and If there is clinical concern, metronidazole may be added. If the unit has high prevalence of multidrug resistant, gram negative organism than carbapenem may be considered. Similarly, if there is high incidence of MRSA, then vancomycin should be considered.
- The duration of antibiotic treatment is usually 7–10 days. Again this is convention without evidence to support Consideration to stopping earlier may be given if the baby is tolerating oral feeds and is clinically improved.
Fluids and Cardiovascular Support
Many infants will be hypovolemic as a result of capillary leak, third spacing and hypoalbuminemia and may require fluid resuscitation. Crystalloid to be given as the initial fluid for volume expansion.
- Consider inotropes if not responding: Dopamine 10 mcg/kg/min then dobutamine 10 mcg/kg/min increasing up to 20 mcg/kg/min
- Correction of thrombocytopenia if platelets < 30
- Correction of anemia with packed cells 15 ml/kg. If there is hemolysis on blood film, do Tk Ag Activation test and give plasma-free red blood cells with low titer
- If there is active bleeding and/or abnormal coagulation profile: give Fresh
Frozen Plasma 10 ml/kg and reassess.
Respiratory Support
Mechanical ventilation is required if there is increasing apnea, oxygen requirements or increasing acidosis.
Correction of Acidosis
The acidemia in NEC is mixed. Correct the respiratory component (hypercarbia and acidosis from hypoventilation) with appropriate ventilatory support. The metabolic component is from hypoperfusion and requires fluid.
Analgesia
If required, commence morphine/fentanyl infusion for adequate analgesia.
Surgery
- Consultation with a pediatric surgeon is essential once the diagnosis has been
· Indications for surgery:
- Identification of a pneumoperitoneum caused by bowel perforation and the presence of necrotic bowel (either may be difficult to diagnose) are two absolute indications for surgical
- Clinical: Failure to respond to optimal medical management as evidence by persistence of 7-point scoring system include severe metabolic acidosis, severe thrombocytopenia, hypotension, hyponatremia, neutropenia, left shift of neutrophils, and positive blood culture. The use of a 7-point scoring system using 7 components that quantitate the presence of metabolic derangement, in conjunction with an infant’s ongoing evaluation by a pediatric surgeon, has been found to optimize the timing of the surgical intervention. It is recommended that this scoring system be used only as an adjunct to careful serial clinical and radiologic evaluation of
– Radiological: Pneumoperitoneum, fixed loop on serial AXR, portal venous gas.
There are two types of surgery performed for NEC: Laparotomy (resection of necrotic bowel, formation of enterostomy and mucosal fistula) and primary peritoneal drainage (PPD). The ability to accurately distinguish NEC from isolated intestinal perforation ( IIP) is important when making a comparison between PPD and laparotomy because mortality associated with NEC is greater than with IIP. A large, single-center, prospective study showed that bowel perforation in infants with severe NEC who were treated with primary
laparotomy fared better than infants treated with a PPD. Conversely, infants with IIP fared better with PPD. Necrotizing Enterocolitis Trial (NET) from the United Kingdom found that 74% of infants initially treated with PPD required a rescue laparotomy. The NICHD NRN trial conducted in extremely low birth weight infants (1000 g), found that laparotomy had an advantage over PPD with respect to the likelihood of survival and better neurodevelopmental outcome at 18 to 22 months of age. Death or impairment occurred in 78% of the drainage group and in 66% of the laparotomy group.17
Stoma Care
Complications related to the stoma are seen in at least half of patients who survive; these include retraction, prolapse, hernia, and wound infection. Although many techniques for ostomy creation exist, none has been shown to be superior in terms of function or complications. Patients with proximal ostomies can have significant issues with fluid losses and resulting electrolyte imbalances, resulting in failure to gain weight, dehydration, and skin breakdown.
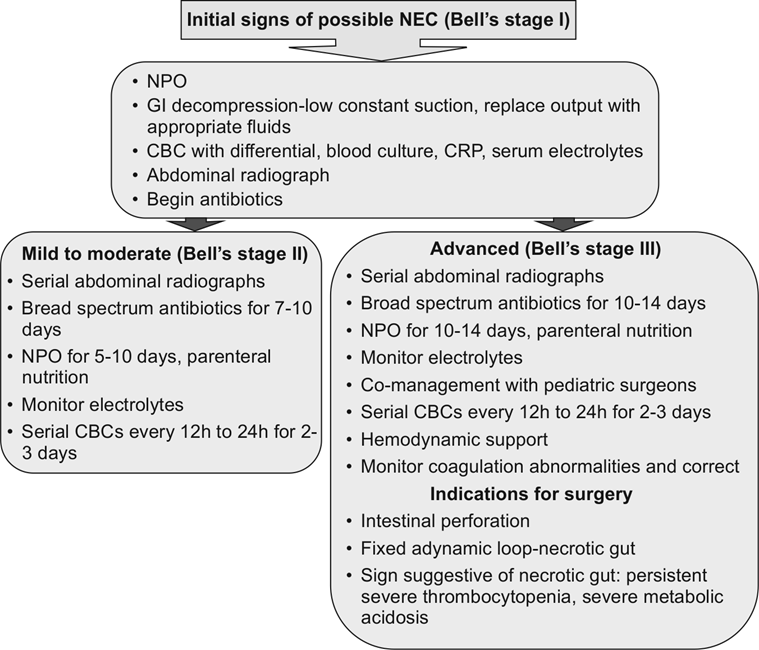
Fig. 22.7 Clinical decision algorithm for NEC
Source: Sharma R, Hudak ML. Clinical erspective of necrotizing enterocolitis.
Past, present, and future. Clin Perinatol 2013;40: 27–51
In patients who have had laparotomy and stoma creation, consideration must be given to the timing of stoma takedown. There is variation in practice between surgeons, with most waiting at least 1 month and some as many as 4 months after the initial operation. Timing of stoma takedown may also be based on patient weight (typically at least 2000 g) and overall medical condition, including adequate nutrition and growth.
MORTALITY
The overall mortality rate in NEC is between 20% and 40% but varies with the severity and extent of gut necrosis. Mortality is inversely related to PMA at birth.
SEQUELAE
Intestinal Sequelae
At discharge many infants have a high prevalence of adverse intestinal sequelae and remain at significant risk of under-nutrition, recurrent illness, gastrointestinal complications, poor growth and recurrent hospitalization. These are associated with:
- Strictures: The most common long-term GIT complication, present in up to 35% of infants who have had They occur in both surgically and medically treated NEC. The most common site is at the junction of the descending and sigmoid colon. Potential indicators of stricture formation are failure to thrive, feeding intolerance, altered stool pattern or bowel obstruction.
- Short bowel syndrome: Seen in 25% of infants that undergo
- Bowel obstruction: In 5% of patients who have had surgery for
- Cholestasis: Secondary to prolonged TPN
- Uncommon sequale: Fistula, abscess, recurrent NEC, malabsorption, enterocyst formation
Neurodevelopmental Sequelae and Growth
Although patients with medically managed NEC are likely to be similar to matched controls in terms of growth and development, survivors of surgically treated NEC are nearly twice as likely to have neurodevelopmental impairment, and developmental delay is seen in approximately 50% of patients. Dysfunction is often shown in vision, auditory, speech, motor, and intellectual delays, as well as problems with interpersonal and social skills.18
INTERVENTIONS AND DIRECTIONS FOR FUTURE RESEARCH
Prediction
These methods use noninvasive indicators, such as profiling of the fecal microbiome, and the identification of the expression of inflammatory proteins from buccal epithelium using buccal swab collection. The determination of
oxidative stress by measuring concentrations of non–protein-bound iron, advanced oxidation protein products, and total hydroxides in cord blood has been reported to be useful in predicting which VP infants are at risk for NEC but require additional validation.4
Prevention: Currently proven strategies for prevention include the use of human breast milk and standardized feeding protocols. These 2 programs are additive and likely even synergistic in their effect on reducing the incidence of NEC; it has been estimated that together, these 2 programs can decrease the incidence of NEC by half.
Probiotics: Probiotics also seem to have promise, and have been described as living microorganisms that are ingested with the intention of colonizing and replicating within the intestinal tract.
KEY LEARNING POINTS
|
The mechanisms of probiotics action seem to be multifactorial; at least in part,their actions can be attributed to modulation of the immune response, strengthening of the intestinal barrier, and production of antibacterial substances. Although exact ingredients vary, these supplements contain potentially beneficial bacteria and yeast, most commonly Lactobacillus, Bifidobacterium, and Streptococcus. Probiotics may aid in normalizing the colonization of the neonatal gut and in improving intestinal function. A Cochrane review of probiotic use identified a reduced incidence of NEC in neonates treated with probiotics as well as a decrease in the mortality. Although these results are encouraging, the specifications are not known regarding strain selection, dose, and duration of supplementation, age to begin therapy, or long-term implications of probiotic use. Although it did not seem increased in the meta-analysis, the risk of sepsis secondary to the probiotic organism is of particular concern in neonates.19


REFERENCES
- Obladen Necrotizing enterocolitis—150 years of fruitless search of the cause.
Neonatology 2009;96:203–210.
- Mizrahi A, Barlow O, Berdon W. Necrotizing enterocolitis in premature infants. J Pediatr 1965;66:697–705.
- Bell MJ, Ternberg JL, Feigin RD. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical Ann Surg 1978;187:1–7.
- Sharma R, Hudak Clinical perspective of necrotizing enterocolitis: Past, present, and future. Clin Perinatol 2013;40:27–51.
- Sharma R, Hudak ML, Tepas JJ 3rd. Impact of gestational age on the clinical presentation and surgical outcome of necrotizing enterocolitis. J Perinatol 2006;26:342–347.
- Sharma R, Tepas Microecology, intestinal epithelial barrier and necrotizing enterocolitis. Pediatr Surg Int 2010;26:11–21.
- Nowicki PT, Caniano DA, Hammond Endothelial nitric oxide synthase in human intestine resected for necrotizing enterocolitis. J Pediatr 2007;150:40–45.
- Peter CS, Feuerhahn M, Bohnhorst Necrotising enterocolitis: Is there a relationship to specific pathogens? Eur J Pediatr 1999;158:67–70.
- Boccia D, Stolfi I, Lana Nosocomial necrotizing outbreaks: epidemiology and control measures. Eur J Pediatr 2001;160:385–391.
- Singh R, Visintainer PF, Frantz ID III. Association of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants. J Perinatol 2011;31:176–182.
- Kennedy KA, Tyson JE, Chamnanvanakij Rapid versus slow rate of advancement of feedings for promoting growth and preventing necrotizing enterocolitis in parenterally fed low-birth-weight infants. Cochrane Database Syst Rev 2000;(2):CD001241.
- McCallie KR, Lee HC, Mayer Improved outcomes with a standardized feeding protocol for very low birth weight infants. J Perinatol 2011;31(Suppl 1):S61–67.
- McGuire W, Anthony Donor human milk versus formula for preventing necrotising enterocolitis in preterm infants: systematic review. Arch Dis Child Fetal. Neonatal Ed 2003;88:F11–14.
- Morgan J, Young L, McGuire Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev 2011;(3):CD001970.
- Berseth CL. Gut motility and the pathogenesis of necrotizing enterocolitis. Clin Perinatol 1994;21:263–270.
- Faingold R, Daneman A, Tomlinson Necrotizing enterocolitis: assessment of bowel viability with color Doppler US. Radiology 2005;235:587–594.
- Kastenberg ZJ, Sylvester KG,The surgical management of necrotizing
Clin Perinatol 2013;40:135–148.
- Dominguez KM, Moss RL, Necrotizing enterocolitis. Clin Perinatol 2012:39:387–401
Patel RM, Denning PW. Therapeutic use of prebiotics, probiotics, and postbiotics to prevent necrotizing enterocolitis. What is the current evidence? Clin Perinatol 2013;40:11–25.
RELATED POST

Empyema Thoracis

Bell Palsy (Peripheral Facial Palsy)

Opportunistic Infections










