Managing Pain in the Neonates
KM Adhikari, Sheila S Mathai, Uma Raju
Part 1 - Spotlight: Neonatal Nutrition
Recent Advances in Pediatrics-25: Perspectives in Neonatology
by Suraj Gupte
INTRODUCTION
Pain is not acceptable to any living being. It has been the endeavor of all medical researchers to alleviate pain and suffering among patients. In this regard, managing pain in the neonates and prevention of pain in newborns has not gained much importance for a long time. There are major gaps in the knowledge on effective pain management in neonates. Complete elimination of pain may not always be possible in neonates as ideal situations to do so may not exist always, however, much can be done by all of us to reduce the amount and intensity of the pain or need for painful procedures. Intervention to reduce pain is important because it is a prudent, ethical expectation and repeated painful exposures can have undesirable consequences.1–13 Due to large number of preterm babies surviving as a result of modern care in highly equipped Neonatal intensive care Units (NICU) all across the globe, there is corresponding increase in the painful interventions and procedures to insure delivery of such care.
There is a much needed balance to be struck between desirable pain relief and avoidance of unwanted and potentially serious adverse effects from pain medications. This decision making is an important challenge area for the neonatologists and all others involved in acute care of these tiny wonders. Not relieving pain in neonates can have significant adverse consequences both in the long run and short term. Though short term consequences disappear fast with reducing pain, the long term consequences of untreated neonatal pain may be detrimental to overall development of the neonate.
ADVERSE CONSEQUENCES
Adverse consequences are highlighted in Table 24.1.
PAINFUL SITUATIONS IN A NEONATE
To effectively alleviate pain in a newborn, it is imperative to first know the situations where pain is likely and pain control measures are warranted. In addition to disease processes and conditions like birth injuries, many diagnostic and therapeutic interventions can result in pain. These scenarios are highlighted in Box 24.1.
RESPONSE OF NEONATES TO PAINFUL STIMULI
The way newborns respond to pain is of importance as it helps in both assessment of painful situations and in measuring the response to therapeutic interventions. These responses are summarized in Table 24.2.

ASSESSMENT OF PAIN IN NEONATES
Favorable pain management mandates effective pain assessment, which can be especially difficult to perform in neonates. The tools that are used to assess the pain should fulfil certain minimum criteria to be effective. Although self reporting of pain is the gold standard for assessment of the site, nature, and severity of pain, it is not precisely applicable in children below 3 years of age. As newborns cannot self report , the tools should be multidimensional, including measurements for both physiologic and behavioral indicators of pain.22–26 Physiologic indicators of pain include changes in heart rate, respiratory rate, blood pressure, oxygen saturation, vagal tone, palmar sweating, and plasma cortisol or catecholamine concentrations. Behavioral indicators include changes in facial expressions (Fig. 24.1), body movements, and crying. It is important to note that these changes may not always be evident in all the sick babies and may be totally absent in neurologically impaired or sedated neonates.
In neonates and infants, surrogate markers are used for pain assessment. Pain is associated with physiological, biochemical, behavioural, and psychological alterations that can be recorded and to some extent, quantified. These changes are summarized in Table 24.2. It is reported that there may be upto 20% increase in the measurable physiological parameters in response to
RAP Special Volume—25: Perspectives in Neonatology
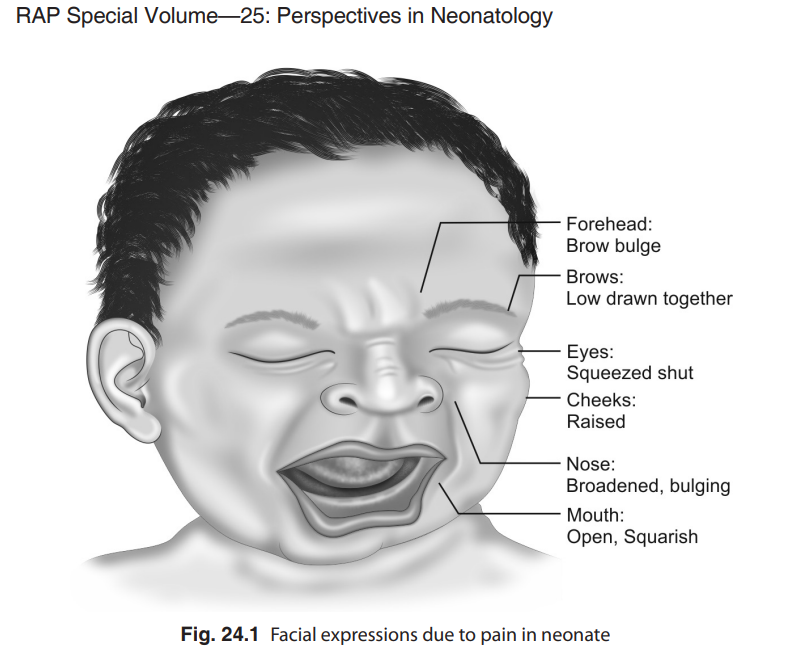
pain. It is relatively easy to measure alterations in most of the physiological parameters without invasive equipment. These measurements coupled with certain consistent behavioral responses are very good indicators of neonatal and infant pain. Of the behavioural changes, the facial expression of the baby is considered the most reliable and consistent indicator with the least interobserver disagreement as well.16 The biochemical changes are definitely the most sensitive quantifiable parameters, however, need to use invasive methods is a major drawback. Due to this reason, they are not routinely used for assessment of pain perception.
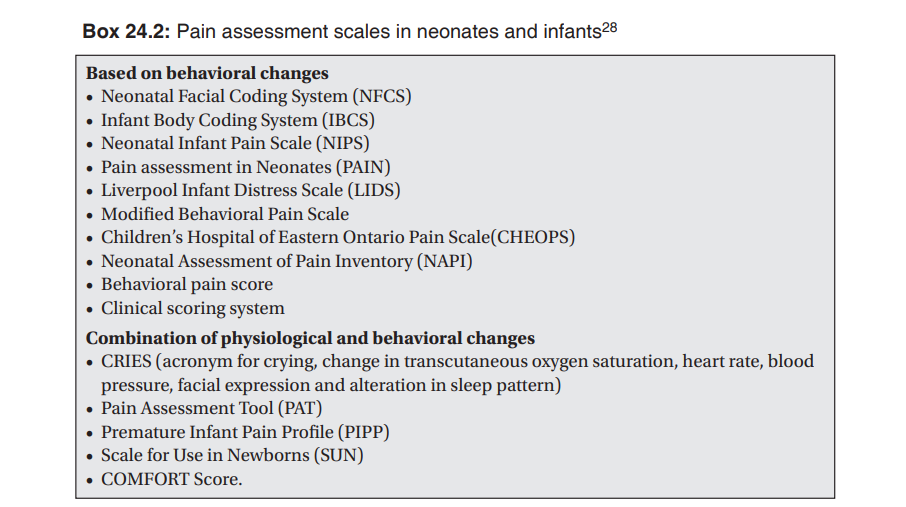
A combination of physiological and behavioral changes provides a better estimate of neonatal and infant pain, as this additive information will increase the objectivity of the assessment. Based on such combination of information, various pain scales have been designed, tested and validated. A detailed description of these scoring systems is beyond the scope of this review. Box 24.2 lists some of the commonly used systems. Clinical discretion is the key while deciding to use these scales in practice, as all these methods of pain assessment are exciting in research settings, and highly sensitive techniques may not always be necessary for effective management of neonatal pain. The mainstays of appropriate management include the professional’s awareness of infant pain, keen understanding of situations wherein pain occurs, sensitivity to the need and genuine effort for controlling pain while adhering the basic principle of ‘primum non nocere’, that mandates actually a generous measure of practical skills and common sense. Although the
physiological and behavioral responses are very sensitive indicators of pain, they have poor specificity and can occur with stress related to disease and discomfort. The responses may also be altered by the physiological state of the baby immediately preceding the painful stimulus, such as the stage of wakefulness27 duration since last feed, restraint techniques used, etc. Notwithstanding all these, assessment of behavioral and physiological responses remain the most readily available, reliable, and feasible method of assessing pain in infants with scope for easy bedside application and no sophisticated equipment or monitoring is required.
Although in recent years, increased interest and research in the assessment of pain and stress in the neonate has occurred, there remains a need to develop a tool to measure pain in pharmacologically paralyzed and severely neurologically impaired infants. Whatever assessment tools are used, continual multidisciplinary training of the staff in the recognition of neonatal pain and in the use of the chosen pain assessment tools should be provided.23
MANAGEMENT OF PAIN IN NEONATES
Prevention and management of pain involves a multipronged strategy. It entails creating an environment using general measures conducive to neonatal care, training healthcare workers to recognize pain in the neonate and several pharmacological and non pharmacological measures. There is a need to come out of barriers that exist regarding management of pain in neonates and small infants (Box 24.3).
General Measures The most obvious strategy would be to reduce the unnecessary painful and stressful conditions in the NICU. Such an approach would include reducing

the number of bedside disruptions in care. Other strategies might include bundling interventions, eliminating unnecessary laboratory or radiographic procedures, using transcutaneous measurements when possible, and minimizing the number of repeat procedures performed after failed attempts.29–30 Box 24.4 suggests general measures that can be adopted in neonatal care units.
Non-pharmacological Measures
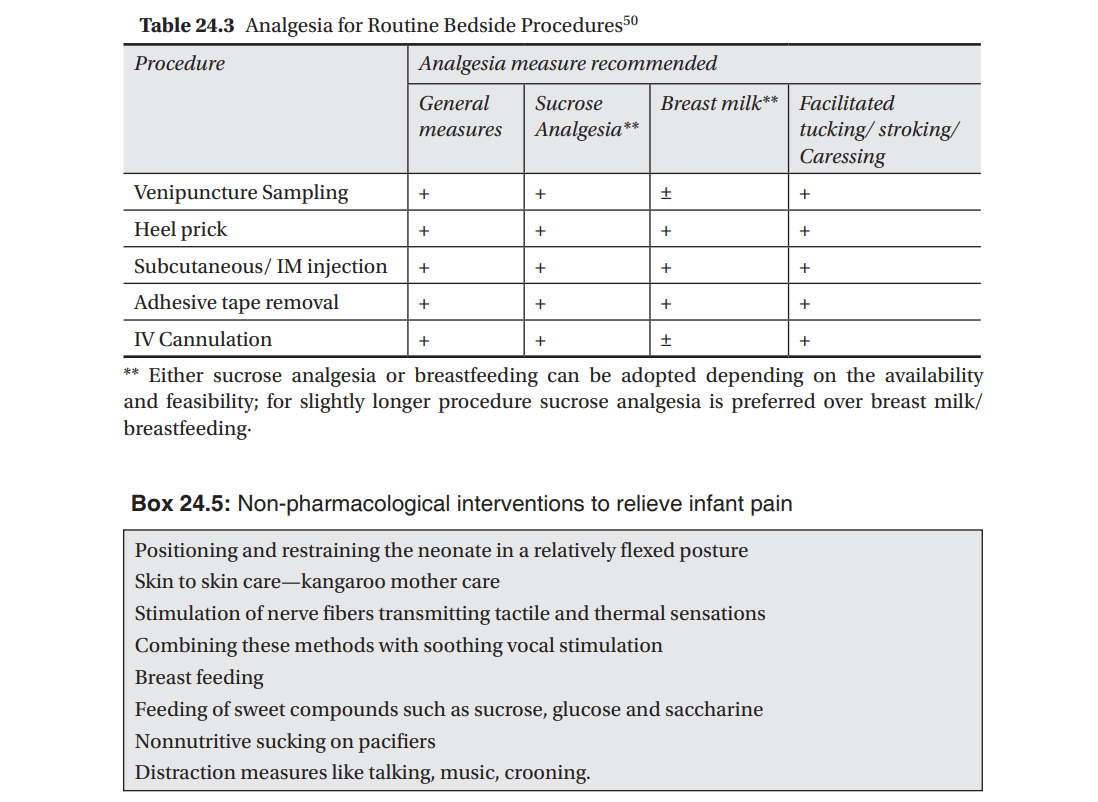
A variety of nonpharmacologic pain prevention and relief techniques have been shown to effectively reduce pain from minor procedures in neonates [Table 24.3]. These include use of oral sucrose/glucose,31–34 breastfeeding,35 non-nutritive sucking,36 “kangaroo care” (skin-to-skin contact),37 alternative female kangaroo care,38 facilitated tuck (holding the arms and legs in a flexed position), swaddling,39 and developmental care, which includes limiting environmental stimuli, lateral positioning, the use of supportive bedding, and attention to behavioral clues.40 These measures have been shown to be useful in preterm and term neonates in reducing pain from a heel stick, venipuncture and subcutaneous injections, and are generally more effective when used in combination than when used alone.39,41 The non-pharmacological measures are summarized in Box 24.5.
Oral Sucrose Administration
Sucrose administration is the most widely studied nonpharmacologic intervention for infant pain management. The soothing, calming, and pain relieving effects of sucrose during painful procedures in neonates are believed to be mediated by the release of endogenous opioid neurotransmitters, such as beta-endorphins. Oral sucrose used alone is appropriate only for pain of
very short duration (2 to 3 minutes), such as heel stick and venipuncture. For treatment of moderate-to-severe pain or when pain is expected to last longer than a few minutes, it should be used in combination with other analgesics or local anesthetics.
Oral sucrose can be administered with a syringe or through a pacifier. Sucrose concentrations of 24% to 50% are recommended as lower concentrations have been found to be less effective. Volumes of 0.1 mL of oral sucrose solution are given to preterm infants of 24 weeks gestational age and upto 2 mL to term infants. The dose must be administered 2 to 3 minutes before the painful procedure, and may be repeated during the procedure. There are no documented adverse effects of oral sucrose in neonates, although hyperglycemia and necrotizing enterocolitis have been postulated as potential effects of repeated dosing. Sucrose has shown to be efficacious and safe in the most mature neonates compared with very preterm neonates. Repeated use of sucrose analgesia in infants younger than 31 weeks gestation was suggested to increase the risk for poor neurobehavioral development and physiologic outcomes in later weeks of life. The age at which oral sucrose no longer produces analgesic effects is unknown. It is believed to be most effective for neonates and not effective for infants older than 6 months. Combining sucrose, oral tactile stimulation, and parental holding
is associated with significantly reduced crying in infants receiving multiple immunization injections. Clinical studies also have shown a pain-reducing effect induced by 30% oral glucose and breastfeeding before venipuncture in newborns.42
A recently published Cochrane meta-analysis of 57 studies involving 4730 infants has shown that sucrose administration is associated with reduced pain scores (PIPP) at 30 and 60 seconds, decrease physiological (heart rate increase) and behavioral indicators of pain (duration of cry, facial action). However, sucrose did not reduce the duration of first cry after heel lance and was ineffective for analgesia for retinopathy of prematurity (ROP) screening. Due to the various concentrations of sucrose used, the meta-analysis was inconclusive about the optimal concentration of sucrose to be used and also the long-term neurodevelopmental effects.43
Breastfeeding and Other Non-pharmacological Modalities Breast milk is also known to be almost as effective as sucrose analgesia in reducing pain for single painful procedure. However, its effectiveness during repeated painful procedures is not established. A Cochrane meta-analysis involving 11 studies shows it to be associated with reduced less duration of cry, less PIPP score, less increase in heart rate in breast fed group. Breastfeeding also involves skin-to-skin contact. One study on breastfeeding documented a decrease in pain scores on the premature infant pain profile (PIPP) and Douleur Aigue Nouveau-ne (DAN) scale even greater than sucrose or holding alone.44 Whereas a Cochrane review found that breastfeeding reduced crying time versus swaddling or pacifiers, its analgesic effects were equivalent to those of sucrose.45 The effects of breastfeeding may be potentiated by multimodal stimulation provided by the touch and smell of the mother and the contained positioning of the infant. Multimodal stimulation or pairing several interventions, such as massage, voice, smell and eye contact, engages more areas of the brain and saturates the sensory channels, thus decreasing painful stimuli.46 Another study by Mathai, confirmed that the use of rocking paired with a pacifier reduced DAN scores more than sucrose or massage alone during heel lancing.47 Consensus statements from the International Evidence- based Group for Neonatal Pain and the Canadian Pediatric Society both recommend the use of swaddling, facilitated tucking, and non-nutritive sucking, both with sucrose or with a pacifier, whenever possible.48 Massage is another comfort measure that can easily be provided to the infant. Although specialized training in massage is useful, neonatal massage done by either a trained mother or a professional, produced weight gains of 6 to 8 g/day above the weight gain rate/day of the control group.49 Massage is thought to increase parasympathetic activity, increase vagal activity, and help produce a more calm organized physiological state. Important in the administration of infant massage is the receptiveness of the provider to the state of the infant. Checking if the infant is engaged with the provider or demonstrating aversion signs, such as looking away, arching, or crying should be monitored before
and during massage. Such no receptive signs must be respected and massage withheld until the baby is ready to engage, to avoid overwhelming the infant.
A multipronged approach using several general and non pharmacological measures for day to day procedures in the NICU causing mild pain to the newborn are recommended (Table 24.3).
Changes in the infant’s environment can also improve comfort. The neonate in the NICU is exposed to many unwarranted visual, auditory, and tactile environmental stimuli that can easily overwhelm the infant’s ability to calm and organize his/her physiological and behavioral state.48,51 The full consequences of multiple overwhelming stimuli are unknown, but visual stimuli have been shown to trigger new NMDA receptors in the visual cortex.52 Providing a quieter environment with an organized day-night cycle promotes sleep, normal circadian rhythms, and reduces heart rate and cortical levels. Batching treatments to provide quiet periods provide time for the infant to regroup his resources.53
Pharmacological Measures to Reduce Pain
While the non-pharmacological measures and topical anesthetic agents can be used for minor procedures, pharmacologic interventions are usually reserved for neonates experiencing moderate-to-severe pain. Although many analgesic agents are approved and available for infants, due consideration must be given to differences in the pharmacokinetics and pharmacodynamics between preterm and term neonates.
Topical Analgesia Topical analgesia using local anesthetics may be used to provide pain relief in bedside procedures, such as arterial punctures, peripherally inserted central catheter (PICC) line placement or lumbar puncture. Tetracaine 4% can be applied locally to the skin 30–60 minutes before the procedure. It should be applied to the intact skin only and not repeated more than once a day to reduce the risk of methemoglobinemia. Its use in combination with non-pharmacological measures provide more effective analgesia.
Systemic Analgesia Neonates, particularly the sick and very premature ones in the NICU are subjected to several procedures which cause moderate to severe pain. These entail the use of pharmacological agents. However, drugs in combination with general and non pharmacological measures would provide more effective relief from pain to these babies. Combination therapy for neonatal analgesia is therefore considered most appropriate (Table 24.4).
Analgesia in Ventilated Neonates While there are definite indications for its use in obviously painful procedures, such as chest tube insertion or circumcision, its use in conditions, such as 320
RAP Special Volume—25: Perspectives in Neonatology
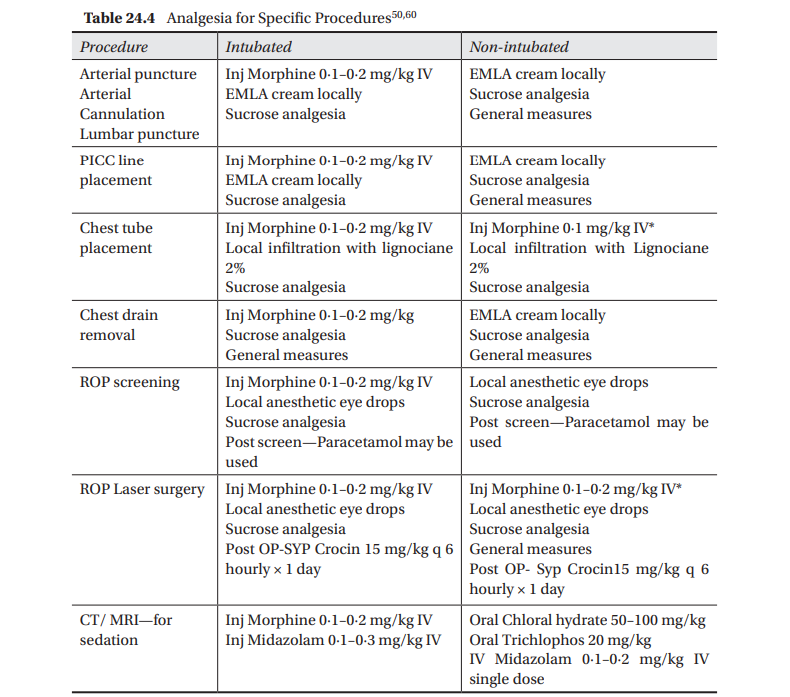
*In non ventilated babies while using Opioids—Watch for apnea/respiratory depression; IV Naloxone should be kept ready and used in case of respiratory depression or apnea (0∙1 mg/kg or 0∙25 mL/kg IV) **Even ventilated patients on opioid infusion during procedures needs additional analgesic measures.
mechanical ventilation has been controversial. Besides being difficult to assess pain in such infants, the reasons usually cited to routinely sedate ventilated neonates include improved ventilator synchrony, improved pulmonary function, and decreased neuroendocrine responses, including cortical, beta-endorphine, and catecholamines. Reasons not to treat include the well-known adverse side-effects of pain medication, especially the opiates, including hypotension from morphine, chest wall rigidity from fentanyl, and tolerance, dependence, and withdrawal from both opiates and benzodiazepines. Additionally, adverse effects, such as death and intraventricular hemorrhage (IVH) are not improved with preemptive treatment.54
Two appropriately powered studies enrolled a total of 1048 neonates and demonstrated no differences in the incidence of severe intraventricular hemorrhage, periventricular leukomalacia, or death outcomes between the ventilated infants who received morphine or placebo infusions.55,56 Pain assessments during tracheal suctioning were unaltered in 1 trial and minimally diminished in the other trial. The largest of these, the neurologic outcomes and preemptive analgesia in neonates (NEOPAIN) Trial, which randomized 898 babies to receive continuous infusion of morphine or placebo, showed an association between morphine and worsening respiratory outcomes, including a significant increase in the duration of ventilation.56 Morphine also contributed significantly to hypotension observed during the first 24 hours of life, particularly in infants of 23 to 26 weeks gestation and those with pre-existing hypotension. It was associated with the use of additional doses of morphine.57 Studies have consistently shown that the use of opioids is associated with a significant increase in the time to reach full enteral feeds.58 Besides these, the NEOPAIN study did not find any added benefit of morphine in terms of either short term outcomes of death or neuro morbidity as assessed by cranial ultrasound or long term neuro developmental outcome. In fact, infants exposed to morphine analgesia may exhibit subtly worse neurobehavioral differences involving motor and popliteal angle on Neurobehavioral Assessment of the Preterm Infant (NAPI) subscales as early as 36 weeks of post-conceptual age (PCA).59 Fentanyl has been used as an alternative and has been found superior to morphine in reduction of PIPP scores, reduction in oxygen saturation spells, in addition to improved cardiovascular stability. However, the studies on its use are limited and caution needs to be exercised due to potential side effects of tolerance and chest wall rigidity.54
In contrast to opiates, trials using midazolam for sedation in ventilated infants has been associated with higher episodes of hypotension, statistically significant higher incidence of adverse neurologic events (death, grade III– IV IVH, PVL) and a longer duration of NICU stay compared to the placebo group .54,61 Hence, use of midazolam in ventilated infants has been generally discouraged. However, if the drug needs to be used as infusion as a for neonates, the following is recommended:
- Doses should be individualized and titrated, and treatment should be limited to a few days
- Continuous infusions are preferred over bolus doses; the maximum dose for continuous infusion is 60 mcg/kg per hour in term neonates, and should be decreased for lower gestational ages
- The maximum for individual bolus doses is 200 mcg/kg, and should be infused over 1 hour
- Doses should be decreased by approximately 30%, if treating concurrently with narcotics
- Do not use in infants who are hypotensive
- Use with extreme caution in infants being treated with fluconazole or other medications (e.g., erythromycin) that interfere with cytochrome P450 3A4 (CYP3A4) metabolism.54
Analgesia during Endotracheal Intubation The experience of being intubated is unpleasant and painful and seriously disturbs physiologic homeostasis.62 A consensus statement from the International Evidence—Based Group for Neonatal Pain concluded that “tracheal intubation without the use of analgesia or sedation should be performed only for resuscitation in the delivery room or for life threatening situations associated with the unavailability of intravenous access.60 Subsequent to this the AAP has recently provided guidelines for premedication for non emergency intubation. The excerpts from this guideline suggest the use of analgesic, muscle relaxant, hypnotic/sedative and a vagolytic as an effective strategy. The use of midazolam is discouraged in preterm due to higher incidences of desaturation, serious cardiovascular and neurological side effects.63
Nonopioid Analgesics Non opioid analgesics are used to treat pain of lesser intensity and as an adjunct to reduce the total dose of opioids (“opioid-sparing” effect). They are valuable in clinical situations requiring mild or moderate analgesia, particularly for the pain associated with inflammation (e.g. for meningitis, thrombophlebitis, cellulitis, necrotizing enterocolitis, or septic arthritis). Nonsteroidal anti-inflammatory drugs (NSAIDs) are effective analgesics in the management of mild-to-moderate pain in children, but they have not been studied adequately in neonates. They are most useful for postoperative pain management .They also are more effective in preventing pain rather than relieving it. This class of drugs includes acetaminophen, acetylsalicylic acid, and nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen and ketorolac. The current availability of intravenous preparations, including ketorolac tromethamine, ibuprofen lysine, and paracetamol, holds promise for clinical utility in critically ill neonates. Unfortunately, limited data are available on the pharmacokinetics and pharmacodynamics for most of these drugs in neonates (with the exception of acetaminophen and ibuprofen). Acetaminophen is considered safe and effective in reducing mild-tomoderate pain in the pediatric population, with limited studies on its efficacy and safety in preterm and severely ill infants. It can be administered safely for a shorter duration of time to relieve mild procedural pain in neonates without the risk of hepatotoxicity. The recommended dose is 10 to 15 mg/kg orally or 20 to 30 mg/kg rectally. Higher doses do not lead to greater analgesic effects. Tolerance generally does not occur, but repeated dosing may result in cumulative hepatic and renal toxicity, although this problem has not been addressed formally in neonates. Acetylsalicylic acid is not recommended for
use in neonates, because it increases the risk of eye syndrome. Ibuprofen and ketorolac have not become standard analgesics in the newborn period because of the potential adverse effects of renal toxicity and platelet dysfunction. Ibuprofen also displaces bilirubin from its binding sites and is associated with an increased risk of gastritis, which limit its use in the NICU.
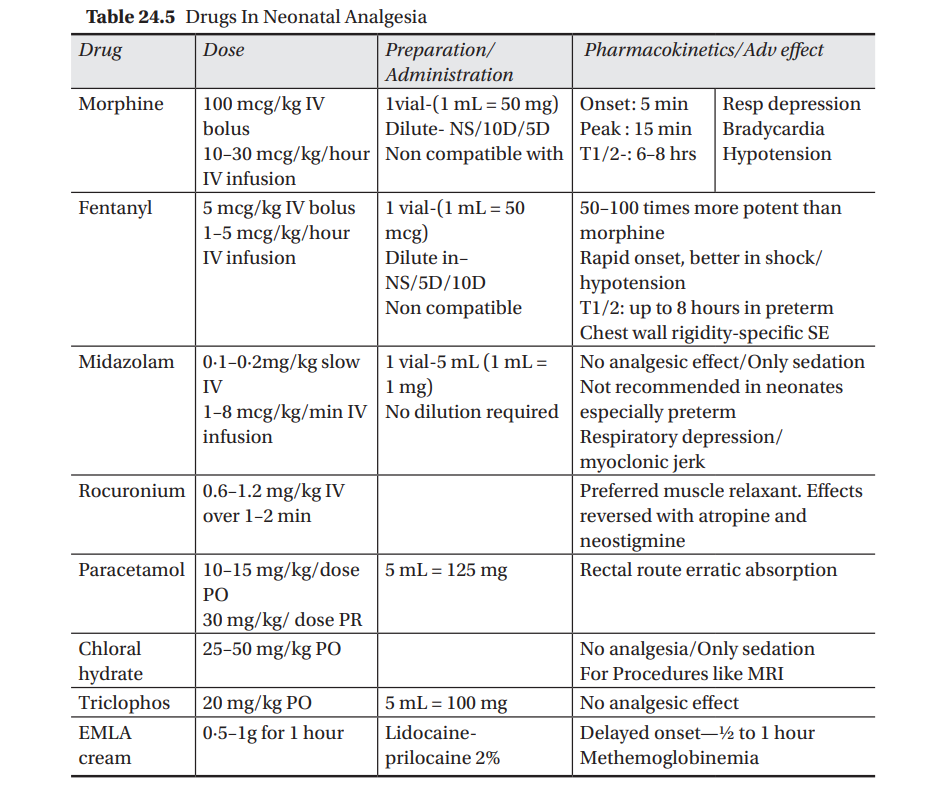
Thus, several pharmacological agents have been used to provide pain relief to newborns. While utilising them , their pharmacokinetics and adverse effects in the neonates have to be kept in mind particularly when dealing with premature and jeopardized babies. The most commonly used agents in the NICU are opoids, mostly morphine and fentanyl, benzodiazapinesmidazolam, acetoaminophen, chloral hydrate and triclophos. Their dosages, preparations and dilution along with common adverse effects are shown in Table 24.5.
SURGERY IN NEONATES AND PAIN MANAGEMENT
Pain is a definite consequence of surgery at every age. This is particularly of significance in the neonate because of the evidence of improved clinical outcomes, including decreased mortality, when adequate pain control is achieved.64,65 Tissue injury, which occurs during all forms of surgery, elicits profound physiologic responses. The more marked these responses to surgery, the greater the morbidities and mortality.66 Thus, minimizing the endocrine and metabolic responses to surgery by decreasing pain has been shown to significantly improve outcomes in neonatal surgery. It would require a multidimensional and team approach to insure adequate analgesia and pain relief in neonates during surgery. Despite fears that analgesics (opiates in particular) may lead to hypotension or respiratory depression and an increase in postoperative complications, such effects have never been shown in randomized, controlled trials. Indeed, postoperative inotrope requirements were decreased by high-dose opioids in neonates after cardiac surgery67 and postoperative respiratory compromise, associated with the pain of a thoracotomy can be relieved by adequate analgesia.68,69
Neonates have immature physiologic and metabolic mechanisms, due to which doses of medications that are effective for the reduction of pain may be close to the doses that cause toxicity. Therefore, the concept of a “balanced analgesia” has arisen, whereby several approaches to pain reduction can be used simultaneously to decrease the dosage required of each medication and, thereby, reduce toxicity. Early and effective pain treatment should be guided by ongoing pain assessment. The developmental pharmacology of the agents used must also be kept in mind. The prolonged and residual effects of intraoperative medications also need to be factored during postoperative decision making and monitoring. Modalities that assist the specialist in postoperative pain assessment may be altered after surgery due to factors like muscle relaxants that completely prevent behavioral pain responses. It is important to note that these residual effects may last for many hours postoperatively.
Preoperative pain should be suitably addressed before surgical interventions. Though there has been little direct investigation of the effects of preoperative analgesia in neonates, it is clear that an infant who is stressed and disturbed, unclothed, hypothermic, overstimulated by noise and light, and already experiencing pain will have elevated basal concentrations of adrenal cortical and medullary hormones and will be susceptible to further stress and complications postoperatively. Wherever appropriate, regional anesthesia is an effective way of controlling intraoperative pain in neonates. Although there are few data specific to the neonate, regional analgesia can provide effective postoperative pain relief in some situations.70,71
For post operative pain control in neonates, opioids can be given by continuous infusion or by regular bolus. Currently completed trials do not show any substantial benefit of continuous infusion of opioids over intermittent dosing, probably because of the long half-life of many of these agents in the neonate.72 Intravenous nonsteroidal anti-inflammatory agents, such as ketorolac and ketoprofen are well established as a means of reducing postoperative opioid requirements in older children and adults,73,74 lacking however, any substantial evidence for their efficacy in the neonatal period. Studies in neonates for postoperative usage of paracetamol seem to be limited to use for circumcision, in which it is ineffective for operative and immediate postoperative pain but decreases later postoperative pain scores at 6 hours.75 Acetaminophen should not be used alone for severe pain, but can be considered for use during the later postoperative period, after minor procedures, or as an adjunct to other measures. There has been little systematic study of ancillary comfort measures in the postoperative neonate. Despite the importance of good pharmacologic treatment, the nonpharmacologic means of reducing pain in neonates discussed previously can also be used postoperatively and should be part of a coordinated effort to reduce the pain and stress experienced by these neonates during the postoperative period.
PAIN MANAGEMENT IN NEONATAL PERIOD: THE WAY AHEAD
There is a growing need to understand the basis of management strategies for neonatal pain and to exit from the apprehensions regarding consequences of being proactive when it comes to managing pain in neonates. Clinicians need to be aware that neonates too have the capacity to feel pain, assessment for cause and severity of pain is possible, pharmacological measures complimented by non-pharmacological interventions have shown definite benefits, and lot of undesirable long-term adverse outcomes can thus be avoided. Neonatal pain relief should be a part of routine nursery and NICU management protocols. The issue of being sensitive to neonatal painful situations should be driven home during undergraduate curriculum and strongly reinforced during post graduate pediatric educations. Life may begin with problems for a neonate, however, the pain and agony associated with it should not go unattended.
REFERENCES
- American Academy of Pediatrics, Committee on Fetus and Newborn. Prevention and management of pain and stress in the neonate. Pediatrics 2000;105:454–446.
- Porter FL, Grunaue RE, Anand KJ. Long-term effects of pain in infants. J Dev Behav Pediatr 1999;20:253–261.
- Anand KJ, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Biol Neonate 2000;77:69–82.
- Bhutta AT, Anand KJ. Vulnerability of the developing brain: neuronal mechanisms. Clin Perinatol 2002;29:357–372.
- Taddio A, Shah V, Gilbert-MacLeod C, Katz J. Conditioning and hyperalgesia in newborns exposed to repeated heel lances. JAMA 2002;288:857–861.
- Oberlander TF, Grunau RE, Whitfield MF, Fitzgerald C, Pitfield S, Saul JP. Biobehavioral pain responses in former extremely low birth weight infants at four months› corrected age. Pediatrics 2000;105:e6.
- Alvares D, Torsney C, Beland B, Reynolds M, Fitzgerald M. Modelling the prolonged effects of neonatal pain. Prog Brain Res 2000;129:365–3173.
- Anand KJ. Effects of perinatal pain and stress. Prog Brain Res 2000;122:117–129.
- Buskila D, Neumann L, Zmora E, Feldman M, Bolotin A, Press J. Pain sensitivity in prematurely born adolescents. Arch Pediatr Adolesc Med 2003;157:1079–1082.
- Maroney DI. Recognizing the potential effect of stress and trauma on premature infants in the NICU: how are outcomes affected? J Perinatol 2003;23:679–683.
- Stevens B, McGrath P, Gibbins S, et al. Procedural pain in newborns at risk for neurologic impairment. Pain 2003;105:27–35.
- Anand KJ. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med 2001;155:173–180.
- Porter FL, Wolf C, Gold J, Lotsoff D, Miller JP. Pain and pain management in newborn infants: a survey of physicians and nurses. Pediatrics 1997;100:626–632.
- Ruda MA, Ling QD, Hohmann AG, Peng YB, Tachibana T. Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science 2000;289:628–663.
- Grunau RE, Oberlander TF, Whitfield MF, Fitzgerald C, Lee SK. Demographic and therapeutic determinants of pain reactivity in very low birth weight neonates at 32 weeks postconceptional age. Pediatrics 2001;107:105–112.
- Anand KJ, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav 1999;66:627–637.
- Fitzgerald M, Beggs S. The neurobiology of pain: developmental aspects. Neuroscientist 2001;7:246–257.
- Grunau RVE, Craig KD. Pain expression in neonates: facial action and cry. Pain 1987;28:395.
- Anand KJS, Carr DB. The neuroanatomy, neurophysiology and neurochemistry of pain, stress and analgesia in newborns and children. Pediatr Clin North Am 1989; 36:795.
- Marx CM. Sedation and analgesia. In: Blumer JL (ed): A Practical Guide to Pediatric Intensive Care. St Louis: Mosby Year Book 1990:292–308.
- Whit Hall R, Anand KJS. Physiology of pain and stress in the newborn. Neo Reviews 2005;6;e61–e68.
- International Association for the Study of Pain, Task Force on Taxonomy. Announcement: modification of pain definition. IASP Newsletter 2001;2:2.
- Walden M. Pain Assessment and Management: Guideline for Practice. Glenview, IL: National Association of Neonatal Nurses;2001.
- Craig KD, Whitfield MF, Grunau RVE, Linton J, Hadjistavropoulos HD. Pain in the preterm neonate: behavioral and physiological indices. Pain 1993;52:287–299. 25. Chiswick ML. Assessment of pain in neonates. Lancet 2000;355:6–8. 26. Franck LS, Greenberg CS, Stevens B. Pain assessment in infants and children. Pediatr Clin North Am 2000;47:487–512. 27. Peters JW, Koot HM, De Boer JB, et al. Major surgery within the first 3 months of life and subsequent biobehavioral pain responses to immunization at later age: a case comparison study. Pediatrics 2003;111:129–135.
RELATED POST

Empyema Thoracis

Bell Palsy (Peripheral Facial Palsy)

Opportunistic Infections









